Ensuring confidence in health instruments
VENTILATOR TESTS, A HEALTH PRIORITY
The instruments in the health area should be evidenced to ensure that they are within parameters of safety, operation and, mainly, accuracy. This is where the ISQ metrology laboratory plays a crucial role, demonstrating the suitability of these important instruments.
Trust
Trust in the various measuring instruments used in different activities or process chains is essential to ensure the efficiency and effectiveness of these same processes. Only in this way is it possible to ensure a correct analysis and consequent decision-making, whenever it applies, raising the firmness in the results obtained.
Different institutions develop in a structured way, through their quality management systems, processes and procedures that allow to evidence the suitability to the use of measuring instruments inserted in their production or service chains. For this, they use the basic principles of metrology as a science of measurement.


Through Metrological Confirmation methodologies, recommended by the different domains of metrology, it
is achieved the inclusion of the different instruments for measuring the various quantities of physics or chemistry, in a national traceability chain, and consequently international, through the Accredited Metrology Laboratories IPAC, making recognizable and binding the quality of the results obtained by these instruments.
Trust in medical equipment
PARTICULARIZING THE AREA OF HEALTH, BETWEEN A VAST AND DIFFERENTIATED SET OF MEASURING INSTRUMENTS WE FIND THOSE THAT BELONG TO THE LIFE SUPPORT GROUP. IN THIS CONTEXT, THE FOLLOWING EQUIPMENT IS AT STAKE:
- Defibrillators, responsible for resuscitation of patients;
- Electrocardiographs and multiparametric monitors, as auxiliary diagnostic means;
- Infusion/infusion systems, used for intravenous administration of drugs or enteric feeding:
- Ventilators, in this phase, are very evidenced, and may play a preponderant role in the maintenance of life in situations of cardiorespiratory deficiency.
Although outside the Life Support group, the electroscalpel used in surgery is also very scrutinized in view of the type of use in clinical and hospital practice.

Assess reliability and accuracy
Some of these instruments are used as means of intervention, others as means of diagnosis, confidence in their use or in the results they emit will be the subject of a decision, certainly involving the quality of human life. It is therefore essential to apply a management mode that ensures its reliability and accuracy.
The processes to be adopted for this purpose should go beyond those applied in preventive maintenance and be based on the principles
of metrology. This measurement science introduces into its methodology the most effective procedures in the analysis of operating parameters and especially accuracy. The development of this metrological activity extends to the entire measurement chain, that is, peripherals and consumables involved in the process of realization, thus evaluating the entire process until contact with the patient and not just the “machine”. At the same time, electrical safety parameters are also analyzed. In
this case, following normative references, it aims to prevent the existence of electrical risks, a component also fundamental in the handling of this type of instruments, essentially with the objective of protecting medical technicians.
The ISQ, through the Metrology Laboratories, in particular Labmetro Saúde in its Electromedicine aspect,
has developed specific methods and procedures for each type of instrument, in accordance with the requirements of the Portuguese Quality System, which are accredited by the IPAC – Portuguese Accreditation Institute.
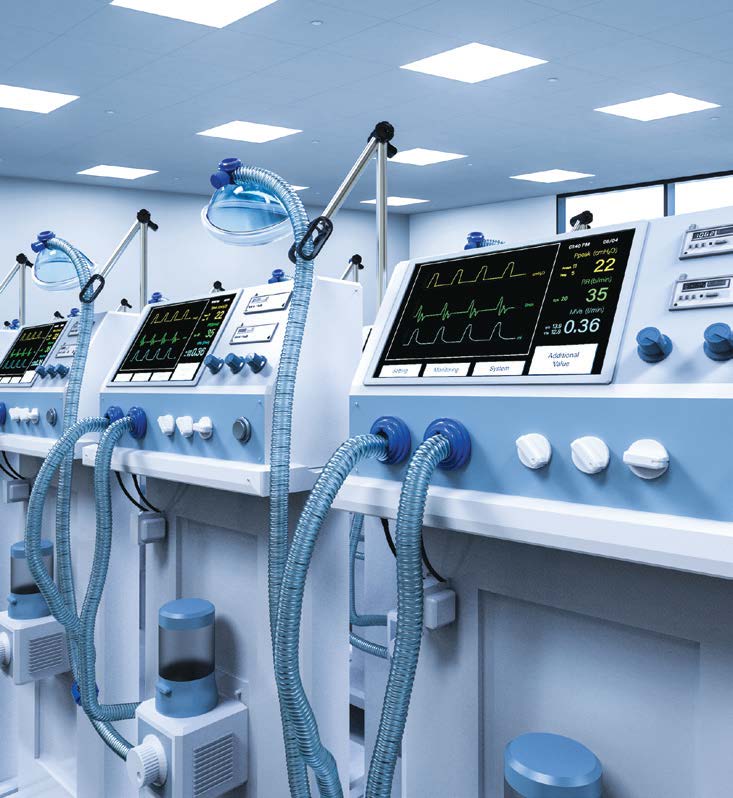
Ventilator trials, a health priority
ISQ performs evaluation and validation tests of life support equipment, such as ventilators, so critical during the pandemic.
Considering the clinical consequences that the COVID-19 infection causes in patients, the placement of life support equipment, such as ventilators, at the service of hospitals has been a priority on the part of the most diverse entities. It is therefore extremely important that, after manufacture, these equipment fulfill the requirements necessary for their function, allowing clinicians to support patients in the treatment of patients.
In this sense, since 2009, ISQ has carried out evaluation and validation tests of life support equipment, namely ventilators, and in the last year there was a high demand for the provision of this type of service. The testing of new ventilators allowed their incorporation in different hospital units, making it possible to improve patient support care.
Faced with the high demand of the last year, the Portuguese industry has dedicated itself to the development of fans that mainly aim to suppress national needs and reduce dependence on other countries. ISQ continues to support the industry, namely with regard to product development and methodologies to support the conformity assessment of fans, development of technical documentation, support for CE marking and placing on the market of this type of equipment.
IPAC Recognition
The IPAC recognition shows the competence of the ISQ Metrology Laboratory in the exercise of
calibration activity or assay necessary for the Metrological Confirmation process to these instruments. Thus, it
is possible in a structured way to demonstrate its suitability, when compared to the
results obtained in metrological operations with the respective acceptance criteria, whether regularly defined by
specific standards for each type of instrument, or by definition of hospital clinical management.



The ISQ-Laboratory of Metrology, through this valence, is positioned as a strong partner of the different clinical and hospital institutions, developing the activity in large national hospitals, as well as in the different public and private medical action institutions or occupational medicine.
EUROPEAN GUIDELINES
Classification of clean rooms
Cleanroom classification is an essential procedure in several sectors and ISQ, through its Environment and Safety Department, develops this activity according to the highest quality standards. In this area, it guides its activity based on the guidelines established by the European Union regarding the classification of clean rooms (EU GMP Guideline) and the ISO 14644-1:2015 standard – “Cleanrooms and associated controlled environments – Part 1: Classification of air cleanliness by particle concentration”. In this context, two areas with classification of clean rooms stand out:

MASK PRODUCTION
With the beginning of the pandemic and the growing need to use respiratory protection masks, there was an increase in the number of companies dedicated to their production, both for the national market and for export. In this sector, ISQ’s role in supporting the implementation of requirements inherent to production conditions stands out. Here, the cleanroom classification and the control of biocontamination are at stake, but also the monitoring of parameters such as temperature, relative humidity and pressure differential
AEROSPACE
In the aerospace sector, strict standards of durability, reliability and performance of satellites and spacecraft are followed. In this context, clean area classification studies were recently carried out in the Infante Project.
It is an R&D project for the development, testing and in-orbit demonstration of technology for a microsatellite intended to serve as a precursor to microsatellite constellations.
+ insight
contribute
As a project initiated by ISQ, insight is open to contributions from everyone who wants to participate and who can bring their vision, scientific studies and reasoned opinion to enrich the themes and the debate.
If your activity is linked to research or the analysis and implementation of measures in the topics discussed here, please contact us using the form attached.






